determine the boiling point of ethyl alcohol What temperature does alcohol boil at
In this post, we will explore the boiling points of alkanes and alcohols. Boiling point is a physical property that indicates the temperature at which a substance changes from a liquid to a gas. It is an important property to consider in various applications, such as the production of fuels, solvents, and pharmaceuticals.
Boiling Points of Alkanes
 Alkanes are a class of hydrocarbons that consist of only carbon and hydrogen atoms. The boiling points of alkanes increase with the increase in molecular size or the number of carbon atoms in the molecule.
Alkanes are a class of hydrocarbons that consist of only carbon and hydrogen atoms. The boiling points of alkanes increase with the increase in molecular size or the number of carbon atoms in the molecule.
For example, methane, the simplest alkane with a single carbon atom, has a boiling point of approximately -164 degrees Celsius (-263 degrees Fahrenheit). As we move up the alkane series, the boiling points gradually increase. Ethane, with two carbon atoms, has a boiling point of approximately -89 degrees Celsius (-128 degrees Fahrenheit). Propane, with three carbon atoms, has a boiling point of approximately -42 degrees Celsius (-44 degrees Fahrenheit).
The trend continues as we move to larger alkanes. Butane, with four carbon atoms, has a boiling point of approximately -1 degrees Celsius (30 degrees Fahrenheit). Pentane, with five carbon atoms, has a boiling point of approximately 36 degrees Celsius (97 degrees Fahrenheit). The boiling point gradually increases with hexane, heptane, octane, and so on.
Boiling Points of Alcohols
 Alcohols are organic compounds that have a hydroxyl (-OH) group attached to a carbon atom. The boiling points of alcohols are generally higher than those of alkanes with similar molecular sizes.
Alcohols are organic compounds that have a hydroxyl (-OH) group attached to a carbon atom. The boiling points of alcohols are generally higher than those of alkanes with similar molecular sizes.
Ethyl alcohol (also known as ethanol), which has two carbon atoms, serves as a good example. It has a boiling point of approximately 78 degrees Celsius (173 degrees Fahrenheit). This is significantly higher than the boiling points of alkanes with the same number of carbon atoms. Methanol, the simplest alcohol with a single carbon atom, has a boiling point of approximately 65 degrees Celsius (149 degrees Fahrenheit).
The presence of the hydroxyl group in alcohols allows for the formation of hydrogen bonds between alcohol molecules. These intermolecular hydrogen bonds contribute to the higher boiling points. The stronger the intermolecular hydrogen bonding, the higher the boiling point of the alcohol.
Alcohols with larger molecular sizes, such as propanol, butanol, and pentanol, have even higher boiling points due to the increasing number of carbon atoms and the potential for more extensive intermolecular hydrogen bonding.
In conclusion, understanding the boiling points of alkanes and alcohols is crucial when it comes to various industrial processes and applications. The boiling points provide insights into the physical properties and behavior of these compounds. As the molecular size and intermolecular forces increase, so do the boiling points. This knowledge is valuable in the fields of chemistry, biology, environmental science, and many others.
If you are looking for Boiling points of alkanes and alcohols | Alcohols | Pinterest | Boiling you’ve visit to the right place. We have 5 Pics about Boiling points of alkanes and alcohols | Alcohols | Pinterest | Boiling like Solved Based on this figure, the boiling point of ethyl | Chegg.com, Boiling points of alkanes and alcohols | Alcohols | Pinterest | Boiling and also What Is the Normal Boiling Point of Ethyl Alcohol. Here you go:
Boiling Points Of Alkanes And Alcohols | Alcohols | Pinterest | Boiling
 www.pinterest.comboiling point points alkanes alcohols chemistry alcohol organic
www.pinterest.comboiling point points alkanes alcohols chemistry alcohol organic
Determine The Boiling Point Of Ethyl Alcohol. - YouTube
 www.youtube.comWhat Is The Normal Boiling Point Of Ethyl Alcohol
www.youtube.comWhat Is The Normal Boiling Point Of Ethyl Alcohol
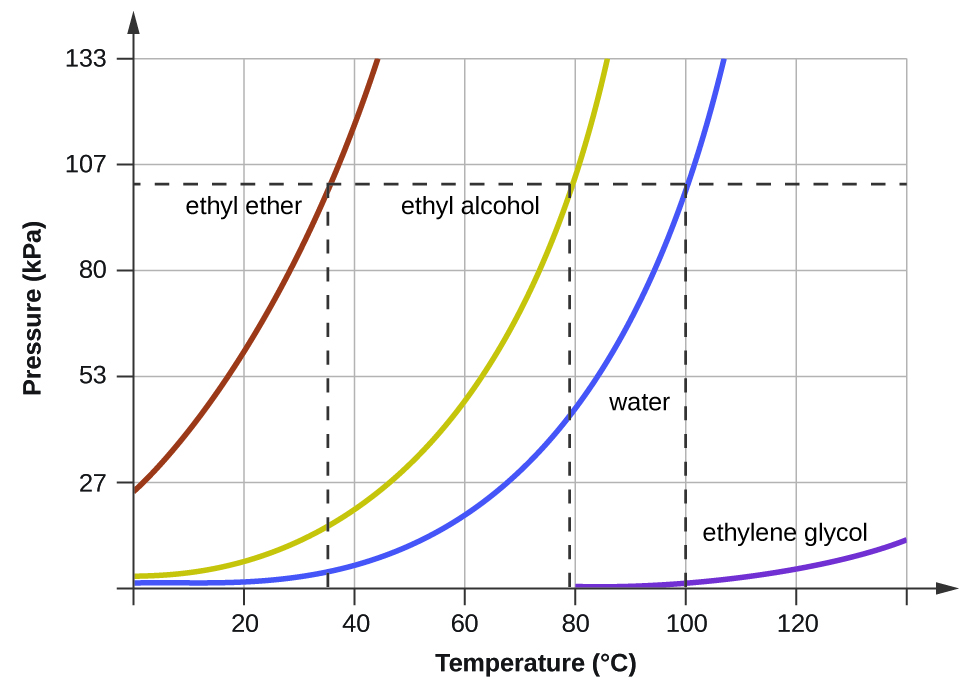 kierra-kcastaneda.blogspot.comWhat Temperature Does Alcohol Boil At
kierra-kcastaneda.blogspot.comWhat Temperature Does Alcohol Boil At
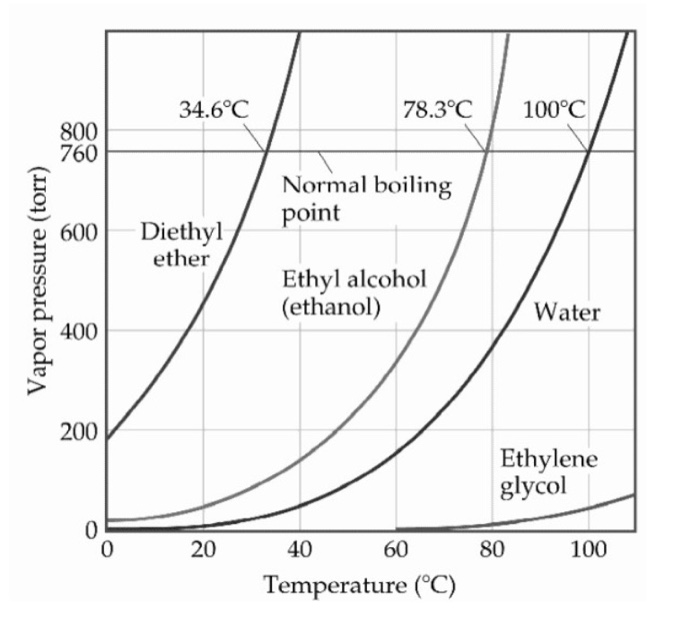 michele1841.blogspot.comboiling ethyl chegg extern
michele1841.blogspot.comboiling ethyl chegg extern
Solved Based On This Figure, The Boiling Point Of Ethyl | Chegg.com
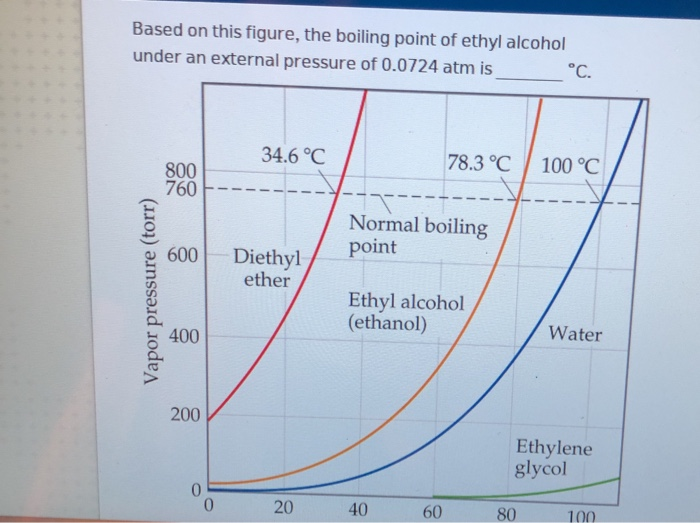 www.chegg.comboiling ethyl atm
www.chegg.comboiling ethyl atm
Boiling point points alkanes alcohols chemistry alcohol organic. Boiling points of alkanes and alcohols. Solved based on this figure, the boiling point of ethyl